For non-neuroscientists
Check out this short presentation on my research for a general public.
This video is part of the initiative BioRoom, a virtual seminar series for biologists around the world. I initiated and co-organized BioRoom seminars during COVID19 lockdown.
For neuroscientists
Interlaminar astrocytes
My current research focuses on interlaminar astrocytes (ILAs), a subset of GFAP+ astrocytes that can be identified in the cerebral cortex by having a soma present in layer I very close to the pia, and long interlaminar processes running into deeper cortical layers. These long processes have been considered to be a predominant feature in the postnatal primate cortex and have been described in many primates and in the lateral cortex of few other mammals (Chiroptera and Scandentia). I am studying ILA evolution, development and functions in the mammalian cerebral cortex, with special emphasis to primates. I am currently working as a postdoc in prof. Martinez Cerdeño lab.
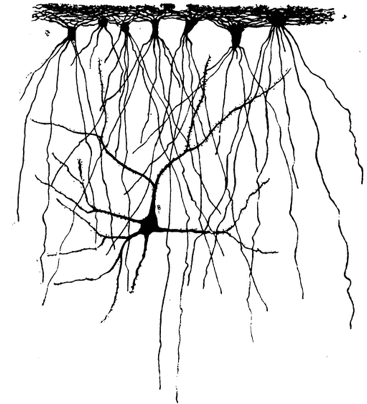
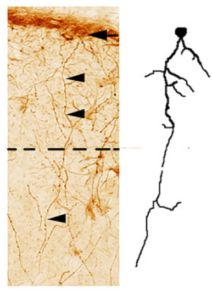
Astrocyte evolution
In a comparative study of ILAs, we have characterized their presence across mammals and we have measured their density and morphological properties across evolution. We have found that ILAs are present in all mammals: rudimentary (with processes not exitying layer I), or typical (with processes crossing layer I-II boundary), but they are more numerous and more morphologically complex in primates. As their processes contact blood vessels and neurites, this work also sugests a potential role for ILAs in blood brain barrier and in the communication among different brain cell types, meninges and cerebrospinal fluids.
Astrocyte development
During my PhD in prof. Mallamaci lab, I extensively studied astrocytes and molecular mechanisms regulating astrocyte differentiation in the cerebral cortex of the mouse.
The aim of my PhD project was to study the role of two transcription factors, Foxg1 and Emx2, in the regulation of mouse cortico-cerebral astrogenesis.
We found that Foxg1 inhibits the early commitment of neural stem cells towards astroglial fates. Furthermore, we showed that Emx2 suppresses the proliferation of astrocyte-committed precursors. Taken together, the results showed that Foxg1 and Emx2 regulation of astrogenesis adds to a variety of early cortical developmental processes regulated by these genes. They also point to those genes as new promising targets to control reactive gliosis and for cell-based design of therapeutics for brain repair.
I am currently interested in the study of ILA prenatal and postnatal development in primates. I am also comparing their development in primates to the one of rudimentary ILAs (layer I astrocytes) in mouse.
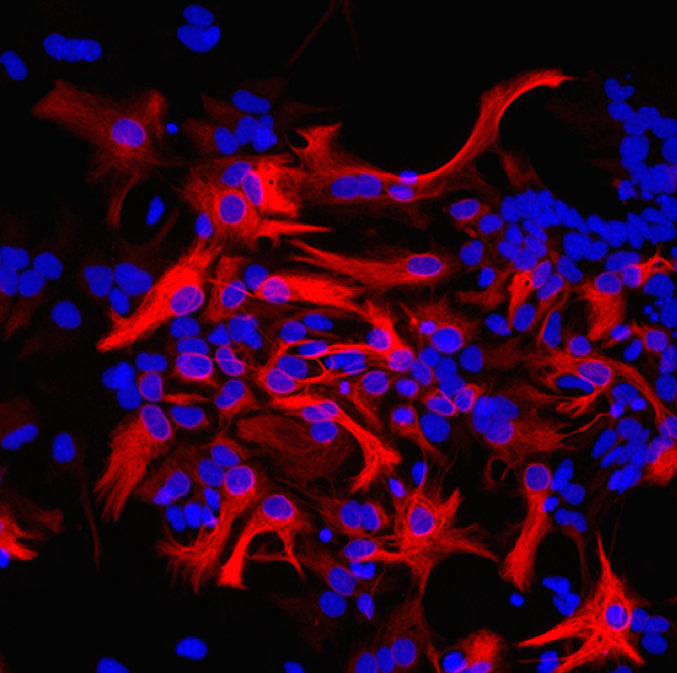
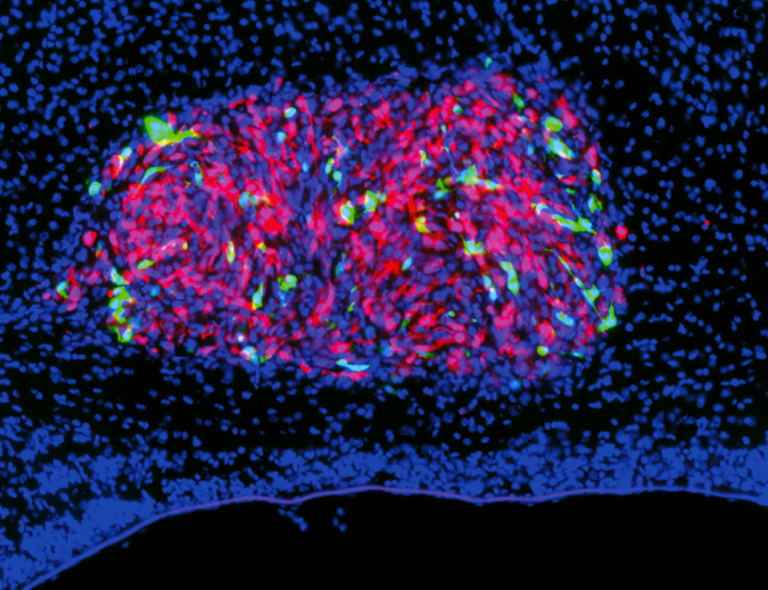
Glioblastoma brain tumor
During my PhD, we translated out findings on the normal astrogenesis to a possible gene therapy to suppress glioblastoma multiforma brain tumor (GBM). No cure is available for this specific type of glioma, which is one of the most aggressive human tumors. We discovered that Emx2 overexpression in GBM patient cell lines induced their collapse in vitro. Emx2 also slowed down the growth of patient cell lines in vivo in the short term, and increased the survival of GBM-transplanted mice in long-term experiments. We found crucial molecular mechanisms underlying Emx2 anti-oncogenic activity. These findings point to Emx2 as a novel, promising tools for therapy of GBM and prevention of its recurrences.